Abstract
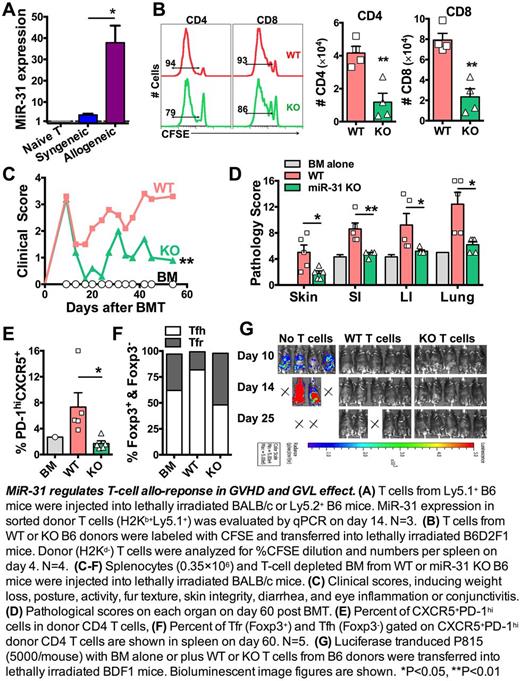
MicroRNAs (miRs) are short, non-coding RNAs that repress gene expression at post-transcriptional level. Certain miRs play critical roles on T-cell responses in inflammation and autoimmunity. To understand the biology of miRs in T-cell allo-responses, we measured expression profiles of miRs in donor T cells in allogeneic vs. syngeneic recipients following bone marrow transplantation (BMT). We found miR-31 was dramatically up-regulated in T cells under alloantigen-driven vs. homeostatic proliferation in vivo(Fig. A). MiR-31 was shown to regulate autoimmunity and cancer. However, its roles in regulating T-cell response remain largely undetermined. Recent study shows miR-31 represses peripheral Treg differentiation in EAE development. In contrast, miR-31 inhibits CD8 T-cell function in anti-virus response. We hypothesize that miR-31 is required for CD4 T-cell pathogenicity in chronic graft-versus-host disease (cGVHD), but is dispensable for CD8 T-cell cytolytic function in graft-versus-leukemia (GVL) activity.
To study how miR-31 regulates T-cell allo-response, we used mice with miR-31 conditional knock out (KO) in T cells (miR-31fl/fl CD4Cre). No differences were observed in the baseline composition of naïve WT (miR-31fl/fl) and miR-31 KO splenocytes. After being transferred into allogeneic recipients, KO T cells had impaired ability to proliferate, reflected by fewer divided (% CFSElow) cells and reduced donor T-cell expansion in recipient spleens (Fig. B). To further evaluate how miR-31 contributes to T-cell pathogenicity in cGVHD, we used a murine model of BMT (B6 → BALB/c), in which low dose spleonocyes can induce scleroderma manifestation. Significant reduction of clinical scores (Fig. C), including improved skin scores and body weight loss, were found in the recipients of miR-31 KO grafts compared to those with WT grafts. This finding was validated in another haploidentical BMT model of scleroderma (B6 → B6D2F1), indicating miR-31 is required for T-cell to induce cGVHD. Consistently, the pathological damage was dramatically reduced in skin, gut and lung in the recipients of miR-31 KO grafts (Fig. D).
Mechanistically, miR-31 deficiency in donor T cells resulted in significantly reduced numbers of CD4 T cells that produce IFNγ, IL-4/5 or IL-17 in recipient spleen and mesenteric lymph nodes. In absence of miR-31, fewer effector and central memory cells were generated from donor CD4 T cells. Further, T cells that are capable of localizing to B-cell follicles (CXCR5+PD-1hi) were significantlyreduced in spleen (Fig. E) and peripheral lymph nodes in recipients of miR-31 KO grafts. Interestingly, among these cells, more foxp3+ follicular T-regulatory (Tfr) but fewer foxp3- follicular T-helper (Tfh) cells were observed in the recipients of KO grafts (Fig. F), indicating miR-31 contributes to the unbalance of Tfr and Tfh cells during cGVHD development. Consistently with the role of miR-31 in regulating Tfr and Tfh cells, we found Blimp1 is the predicted targets of miR-31, which is critically involved in the homeostasis of Tfr and Tfh cells. On donor B-cell compartment, lower levels of CD86 and MHC-II expression were observed in the recipients of miR-31 KO grafts. In addition, germinal center B cells and plasma cells were also reduced in those recipients, indicating miR-31 indirectly regulates B-cell activation and differentiation through controlling T-cell pathogenicity.
For translational purpose, we evaluated how miR-31 impacts T cell-mediated GVL effect. Using B6 → BDF1 BMT model, we consistently found attenuated skin manifestation in recipients of KO T cells. Importantly, in the ongoing experiment, miR-31 KO T cells were capable of controlling P815(mastocytoma) growth as efficiently as WT T cells (Fig. G), suggesting miR-31 may be dispensable for T cells to mediating GVL activity after allogeneic BMT.
Taken together, the current work reveals that miR-31 plays pleotropic roles in enhancing CD4 T-cell pathogenicity in cGVHD. MiR-31 represents a promising therapeutic target for the control of cGVHD while sparing GVL effects after allogeneic hematopoietic stem cell transplantation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal